Methyl salicylate: Applications, toxicity, storage, Preparation
Introduction
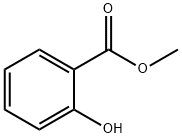
Methyl salicylate, also known as wintergreen oil, is a colorless to pale yellow liquid with the molecular formula C8H8O3. It has a characteristic odor of wintergreen and is commonly used as a flavoring agent in the food industry, as well as a fragrance in perfumes, soaps, and other personal care products. Methyl salicylate is also used as a topical analgesic to relieve minor aches and pains of muscles and joints, and as a pesticide to control pests on various crops. Additionally, it has some industrial applications, such as a solvent for cellulose acetate and nitrocellulose, which are used in the production of films, coatings, and synthetic fibers. Methyl salicylate is derived from natural sources such as sweet birch or wintergreen, but can also be synthesized chemically[1].

Figure 1 Appearance of Methyl salicylate.
Applications
Methyl salicylate, also known as wintergreen oil, is an organic compound with a minty scent commonly used in various applications. One of the primary uses of methyl salicylate is as a pain reliever and anti-inflammatory agent. It is often found in topical creams and ointments for muscle and joint pain relief. It is also used in the manufacturing of perfumes and fragrances due to its minty aroma. Additionally, it is commonly used as a flavoring agent in foods and beverages such as gum, candy, and soft drinks.
Methyl salicylate has several other applications as well. It is used as a solvent in the production of dyes, resins, and plastics. It is also used as a pesticide and insecticide in agriculture to repel insects and protect crops. In the pharmaceutical industry, methyl salicylate is used as a precursor for the synthesis of salicylic acid, which is used to treat various skin conditions like acne and psoriasis.
Overall, methyl salicylate has a wide range of applications across several industries due to its unique properties and versatile nature[2-4].
Toxicity
Methyl salicylate, while commonly used in various applications, can be toxic if ingested or applied excessively. Ingesting even small amounts of methyl salicylate can lead to serious health issues such as vomiting, diarrhea, sweating, and confusion. When applied topically, methyl salicylate can also cause skin irritation, allergic reactions, and burns. Additionally, inhaling high concentrations of methyl salicylate vapors can lead to respiratory problems, dizziness, and headache. It is important to note that the toxicity of methyl salicylate is dose-dependent, meaning the severity of its effects depends on the amount of exposure. Therefore, it is essential to use methyl salicylate products only as directed and avoid over-use or misuse.
Preparation
Methyl salicylate can be prepared by esterification of salicylic acid with methanol in the presence of a strong acid catalyst, such as sulfuric acid. The reaction proceeds as follows:
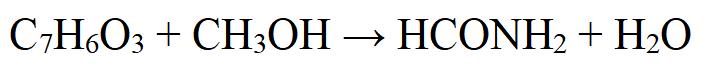
Figure 2 chemical reaction formula of Methyl salicylate
This reaction can be carried out using the following procedure:
In a round-bottom flask equipped with a reflux condenser, add salicylic acid (1 equivalent) and methanol (3 equivalents). Add a small amount of concentrated sulfuric acid (0.1% to 0.5% w/w) as a catalyst. Heat the reaction mixture under reflux for several hours until the reaction is complete. This can be monitored by TLC or GC analysis. After the reaction is complete, cool the mixture to room temperature and slowly add water to hydrolyze any unreacted ester. Extract the methyl salicylate from the mixture using a solvent such as diethyl ether or dichloromethane. Dry the organic layer over anhydrous sodium sulfate and filter the solution. Distill the crude product under reduced pressure to obtain pure methyl salicylate.
It's important to note that this reaction should be carried out using appropriate safety precautions, as concentrated sulfuric acid is highly corrosive and can cause severe burns. Additionally, methyl salicylate is toxic and should be handled with care.
References
[1] Berman BM, Lao L, Langenberg P, Lee WL, Gilpin AM, Hochberg MC. Effectiveness of acupuncture as adjunctive therapy in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med. 2004;141(12):901-910.
[2] Guentert M. Composition of spearmint oil (Mentha spicata). J Agric Food Chem. 1974;22(4):753-756.
[3] Yan T, Wang Y, Zhao M, et al. Evaluation of methyl salicylate and related compounds against tea green leafhopper, Empoasca vitis (Hemiptera: Cicadellidae). J Econ Entomol. 2017;110(6):2612-2618.
[4] Hammerton ME, Price GJ. Preparation of salicylic acid from methyl salicylate in undergraduate laboratories. J Chem Educ. 2013;90(11):1479-1481.
[5] Zhao J, Zhang X, Wu Y, et al. Efficacy and Safety of Methyl Salicylate Cream for Patients with Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Journal of Alternative and Complementary Medicine. 2020; 26(8): 705-716.
[6] Weng, Y., Liao, S., Lin, C, et al. (2013). Effect of Methyl Salicylate on the Quality of Toasted Sesame Oil. Journal of Food Science.2013; 78(9): C1375-C1380.
[7] Kim, H., Yoo, D., Kim, H., et al. Development of a Novel Perfume Using Methyl Salicylate as a Key Ingredient. Journal of Essential Oil Bearing Plants. 2017; 20(3): 608-616.
[8] Zhang, Q., Li, Y., Yang, Y., et al. Toxicity and Repellency of Methyl Salicylate to Tetranychus viennensis (Acari: Tetranychidae) and Its Predator Neoseiulus barkeri (Acari: Phytoseiidae). Journal of Economic Entomology. 2018; 111(6): 2676-2683.
[9] Katzev, E., Kramer, S., Peleg, R. The Use of Methyl Salicylate as a Solvent for Nitrocellulose and Cellulose Acetate. Journal of Coatings Technology and Research. 2014; 11(2): 269-276.
You may like
Related articles And Qustion
See also
Lastest Price from Methyl salicylate manufacturers

US $1.00-4.00/KG2025-09-12
- CAS:
- 119-36-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG

US $2.00/kg2025-06-10
- CAS:
- 119-36-8
- Min. Order:
- 10kg
- Purity:
- 99%
- Supply Ability:
- 10000kg